Featured
- Get link
- X
- Other Apps
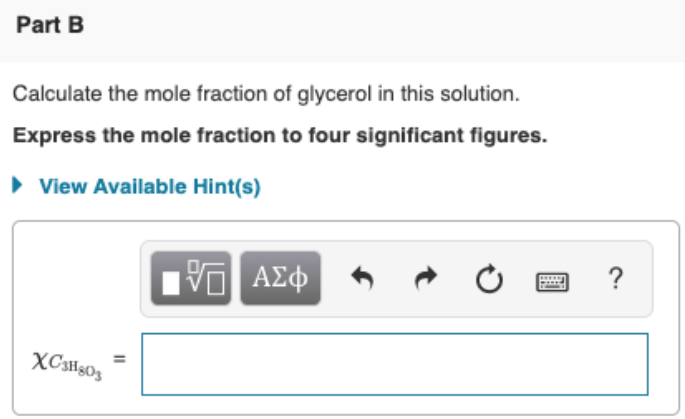
Calculate The Mole Fraction Of Glycerol In This Solution
Calculate The Mole Fraction Of Glycerol In This Solution. It was determined that the volume of water needed to do this was 998.8 ml. Calculate the mole fraction of nacl and h 2 o, if 0.010 moles of nacl is dissolved in 100 grams of pure water.
Calculate the mole fractions of the components of the solution composed by. Answer:mole fraction of glycerol =1mol/6mol=0.167 explanation:this is the right answer to the question please mark me brainliest johnmo799 johnmo799 2 minutes ago (m(water) = 18 , m (glycerol) = 92).
13.6.2Pe Expand_More Science Chemistry Chemistry:
It was determined that the volume of water needed to do this was 998.8 ml. Determine the molality and mole fraction of sucrose if a solution is prepared by dissolving 100.6. Calculate the mole fractions of the components of the solution composed by `92g` glycerol and `90g` water ?
Calculate The Mole Fractions Of The Components Of The Solution Composed By.
Example 1 a tank is charged with a mixture of 1.0 x 10 3 mol of oxygen and 4.5 x 10 3 mol of helium. Jayant kumar, 8 years ago grade:11. Click here👆to get an answer to your question ️ mole fraction of c3h5(oh)3 in a solution of 36 g of water and 46 g of glycerine is:
The Sample Was Created By Dissolving A Sample Of C3H8O3 In Water And Then Bringing The Volume Up To 1.000 L.
View solution > view more. That is c three h 803 by mass. The molecular weight of water = 18.0153 grams per mole.
Answer:mole Fraction Of Glycerol =1Mol/6Mol=0.167 Explanation:this Is The Right Answer To The Question Please Mark Me Brainliest Johnmo799 Johnmo799 2 Minutes Ago
Video answer:here we have a glycerol water solution and is 40% glycerol. Mole fraction of nacl is 0.018 and the mole fraction of water is 0.982. We can calculate the moles of glycerol in the solution which is equal to 40 g, multiplied by one mole, divided by 92.1 g.
Calculate The Mole Fraction Of Each Gas In The Mixture.
3 show answers another question on sat. Please note that mole fraction represents a fraction of molecules, and since different molecules have different masses, the mole fraction is different from the mass fraction. Calculate the mole fraction of ethylene glycol (c2h6o2) in a solution containing 20% of c2h6o2 by mass.
Comments
Post a Comment